Inorg. Chem. 2020, 59, 1996-2004, DOI: 10.1021/acs.inorgchem.9b03363
Authors: Santosh V. Mohite, Shanhu Liu*, Ruimin Xing, Bingyue Li, Sanjay S. Latthe, Yong Zhao, Xiying Li, and Liqun Mao,
College of Chemistry and Chemical Engineering, Henan University, Kaifeng, 475004, P. R. China
E-mail:liushanhu@vip.henu.edu.cn
Considering the environmental issues and depletion of fossil fuels, there is an urgent need to search an alternative energy carrier, which must be clean, renewable, and portable. Water electrolysis is one of the promising methods employed for the generation of highly pure hydrogen gas (H2) and oxygen (O2) without carbon emission via direct splitting of water molecules. However, the substantial energy consumption cannot be ignored due to the sluggish reaction kinetics of hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which generally requires high overpotentials. As known, the efficiency of hydrogen production via electrochemical water splitting is highly dependent on electrocatalysts, which are expected to afford high current at low overpotentials. Considering that, alkaline water electrolysis is more popular due to the lost-cost, highly durable and earth-abundant bifunctional catalysts with low overpotentials are desirable in the industrial fields.
Recently, Liu’s group developed a novel carbon-based CoP hybrid with spatial compartmentalization of CoP nanoparticles (NPs) in P-doped dual carbon shells, which is achieved via a cheap Co−glycerate-template strategy. Benefitted from the uniform atomic blending of Co2+ ions in the Co−glycerate precursors, CoP NPs in situ formed in the confined space with NaH2PO2 as phosphorus source during the annealing process; meanwhile, glycerate suffered carbonization and transformed into P-doped dual carbon shells during the annealing process, including interior thin carbon coating, closely encircled CoP NP, and peripheral hollow carbon sphere loading a lot of CoP NPs. Not only does spatial compartmentalization of CoP NPs avoid the aggregation and expose more active sites, but also P-doped dual carbon shells improve the conductivity and durability of the catalyst. As expected, the optimized hybrid exhibits outstanding electrocatalytic activities in alkaline media, such as hydrogen evolution reaction (HER) overpotential of 101 mV, oxygen evolution reaction (OER) overpotential of 280 mV, and a low cell voltage of 1.66 V to deliver a current density of 10 mA cm−2. Moreover, durability and stability are greatly improved under harsh electrochemical conditions.
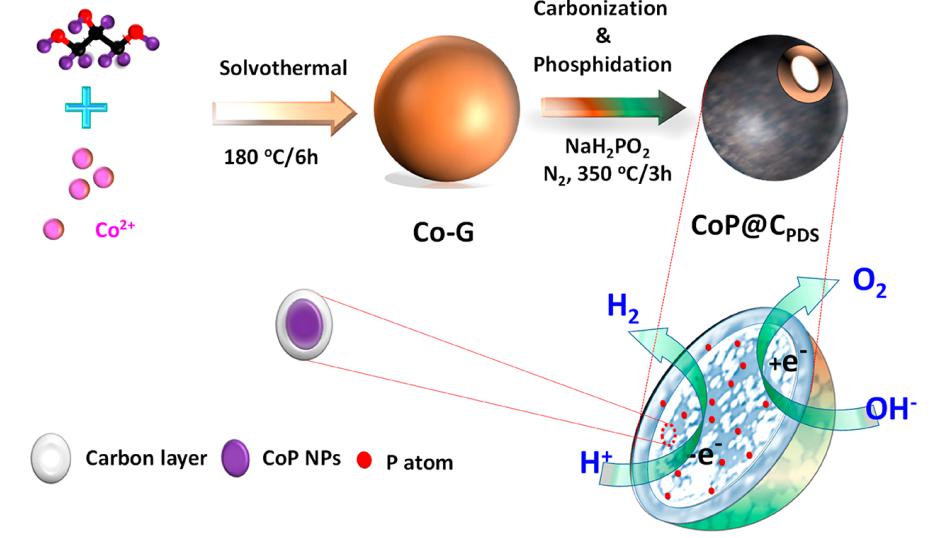
Figure 1. Schematic illustration for CoP NPs encapsulated in P-doped dual carbon shells
Santosh V. Mohite, a postdoctoral student of 2018, is the first author.
Associate Professor Shanhu Liu is the only corresponding author,
Henan University is the exclusive holder.
This work was supported by the National Natural Science Foundation of China (21950410531) and Science Technology Research Project of Henan Province (182102410090).
LINK: https://dx.doi.org/10.1021/acs.inorgchem.9b03363.
Inorg. Chem.: SCI, top, IF= 4.85


